Ozone depletion potential or ODP is a very crucial matter in the recent era. In this article, we will learn, what is ODP, why ozone layer depletion happens, root causes, effects, etc. Let’s explore the ozone depletion potential!
Ozone Depletion Potential (ODP)
Let’s try to understand ozone depletion potential. The advancements in the technologies come up with some price sometimes. Ozone layer depletion is one of the most critical examples of it. The Ozone layer is a protective layer in the earth’s stratosphere and this layer is now depleted. There are various reasons for the depletion of the ozone layer. It may be many reasons, like,
- Various toxic gasses
- Refrigerant like chlorofluorocarbons,
- the exhaust gases from industries and vehicles, etc. are taking part in this
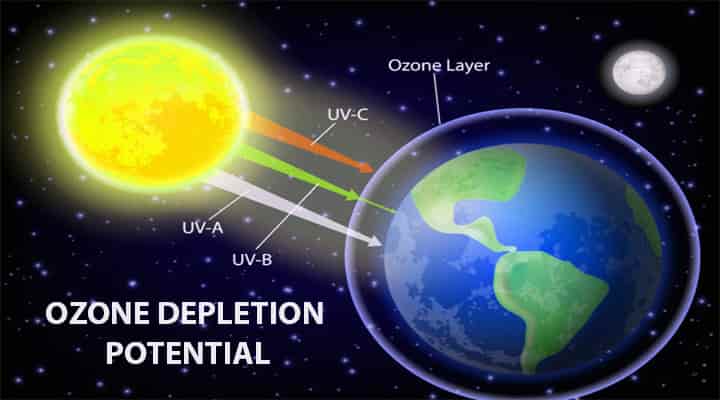
Depletion of the Ozone layer has long-lasting effects, that is why the depletion of the ozone layer should be addressed. There are some rules and protocols made after realizing the threat because of the depletion. Let’s know more about the depletion, causes, and effects on life on earth.
Ozone Layer Basics
Before going towards the ozone depletion potential let’s know more about the Ozone layer in a brief.
- The ozone layer exists in the stratosphere that is the lower area of the earth’s atmosphere.
- It has a high concentration of O3 layer.
- The layer can be found above 15-35 kilometers above the earth but its thickness varies according to seasons and geography.
Back in 1913 Charles Febry and Henri Buisson discovered the ozone layer. They found the radiation rays from the sun were getting absorbed, later scientists developed the Dobson unit to measure the amount of ozone overhead.
Why the ozone layer is so important?
In simple words, the ozone layer is a protective layer that protects the life,
- This layer protects the earth from the harmful UV light rays from the sun.
- There are huge threats to the UV light coming from the sun. Even if the small ozone layer is depleted it will increase the UVB light intensity levels.
- It can cause diseases like skin cancer.
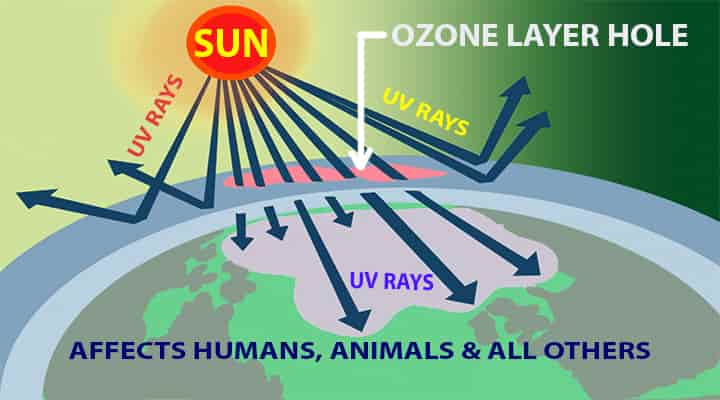
A small percentage of the hole has been seen in Antarctica regions. Right now, there is no direct evidence of damages due to the small percent of the hole. If this continues the levels of UV rays will rise up.
- It could magnify mainly the negative effects on the human body like sunburn, skin cancer, and cataracts, and many more.
- It could magnify the production of vitamin D a little bit which is good for health. But the negative effect is much greater than this vitamin production.
- Not only the humans there are effects on animals as well as crops.
In November 2011 report at the institute of zoology in London found, cases of widespread evidence of epidermal damage commonly associated with acute and severe sunburn. These are mainly due to rising levels of UV lights.
- Crops such as rice which has cyanobacteria for the retention of Nitrogen.
- The cyanobacteria are sensitive to sunlight and thus it can damage the crops.
- If the depletion continues the widespread effects can be seen in coming times.
These are the reasons why the ozone layer is so important right now. Let’s know about the Ozone depletion potential.
Ozone Depletion Potential Basics
The term ozone depletion potential, in simple words, is the thing of the earth’s protective ozone layer. It is caused due to the release of chemical compounds in the atmosphere mainly, bromine and chlorine. Basically, the Ozone depletion potential (ODP) is a measure of how much a chemical can cause damage to the ozone layer of a similar mass of trichlorofluoromethane (CFC-11). CFC-11.
The ODP number 1.0 is used as a base for measuring the depletion potential. So, the higher the number is more is the damage to the ozone layer by a chemical. Different chemical compounds have different ODP numbers. Just like the R-22 has an ODP of 0.05. On the other hand, bromine has a tendency to react with ozone and that is why brominated substance has high ODP range. It sometimes goes up to 15 from 5.
Ozone Depletion Potential Numbers
Here is the common list of compounds and their ozone-depleting potential numbers.
| COMPOUND | R-NUMBERS | ODP NUMBER |
| Ammonia (NH3) | R-717 | 0 |
| Trichlorofluoromethane | R-11 | 1 |
| Tetrafluoroethane-1,1,1,2 | R-134a | 0.000015 |
| Chlorodifluoromethane | R-22 | 0.05 |
| Dichlorodifluoromethane | R-12 | 1.00 |
| Chlorotrifluoromethane | R-13 | 1 |
| Bromochlorodifluoromethane | R-12B1 | 7.9 |
| Carbon tetrachloride | R-10 | 0.82 |
| Nitrous oxide | R-744A | 0.017 |
So, basically, the ozone-depleting substances contain,
- chlorofluorocarbon,
- carbon tetrachloride,
- hydrochlorofluorocarbons, and
- methyl chloroform.
The chlorofluorocarbon causes the highest risk of ozone layer depletion. Now we have known about the various chemicals and their ODP numbers. Now let’s know how exactly these chemicals cause ozone layer depletion.
Causes of Ozone layer depletion
There are two types of causes for ozone depletion potential,
- Natural causes
- Man-made causes.
Natural causes
The causes of ozone layer depletion are man-made. There are some natural causes like sun-sports and stratospheric winds but these are not much effective. Their effects are temporary and only cause 1-2% of depletion.
Man-made causes
If we look towards man-made causes there are causes like the release of chlorine and bromine gases. The man-made compounds like,
- chlorofluorocarbons (CFCs),
- methyl chloroform,
- carbon tetrachloride, and
- HCFCs (hydro-chlorofluorocarbons.
As we have seen in the above sections these substances are categorized as ozone-depleting potentials. You may be thinking like, these gases would be washed out in the forms of rains. But that’s the main problem these gases don’t get washed in the form of rains; they remain in the atmosphere for a long time.
- These gases travel in the atmosphere and carried to the stratosphere.
- In the stratosphere, the UV lights break these gases and they release Chlorine and bromine.
- The Chlorine and Bromine destroy the ozone layer atomic structure.
- Even one chlorine atom can break about more than 100000 molecules of ozone.
- If that’s not enough the bromine is 40 times more destructible than chlorine.
Ozone-depleting substances
Hydrofluorocarbons (HCFCs)
These are somewhat less harmful than Chlorofluorocarbons. The HCFCs were serving the purpose of ozone layer depletion replacing the CFCs.
Chlorofluorocarbons (CFCs)
These are the most harmful substances responsible for ozone layer depletion. You can think of its impact as the CFCs account for more than 80% of ozone layer depletion. One of the reasons for this much percentage is that they are used in lots of applications. It was used as a coolant in refrigerators, air conditioners at home as well as in cars & other various industries. They have been extensively used in the cars and household applications manufactured prior to 1995.
Halons, Carbon tetrachloride
These are mainly found in fire extinguishers.
Methyl Chloroform
These are used in the industries for cold cleaning, vapor degreasing works. Now we know about the causes of ozone layer depletion. The effects are also a future threat let’s know more about them.
Effects of Ozone Layer Depletion
Environmental damages
- Lots of crops and plants are sensitive to UV light rays. Even economic crops like rice can be affected because of the UV light rays.
- It causes the minimal growth of plants, photosynthesis problems, and flowering.
- Apart from the economic crops the forests also bear the cost of ozone depletion.
Human health issues
The ozone layer depletion will cause the UV rays to come directly to the earth.
- Human skins are sensitive and can receive heavy damage due to strong UV rays.
- The most common problems due to UV light rays are skin cancer, cataracts, sunburns, quick aging, and also weakened immune systems.
Aquatic life and effects on animals
The planktons are mostly affected by ozone layer depletion. The planktons are the organisms found in water. Planktons include plants and animals which can propel themselves against the wind. In the food chain of aquatic animals, they are on the top. If their numbers are reduced the marine food chain will be disrupted. Also, certain species of fish are prone to UV light rays.
Effects on materials
The materials are also affected by the harmful UV rays. Materials like plastics, wood, fabrics, rubber can be degraded because of UV radiation. So how we can stop or at least decrease the rate of depletion? There are some ways which we can employ to protect the ozone layer. Also, the scientist is searching for the alternatives for the gases that cause the ozone depletion.
Solutions for ODP
Natural ways over pesticides
On the farm, various pesticides are used to get rid of pests and weeds. This use should be limited and natural ways of getting rid of weeds and pests should be encouraged. The chemicals added to the pesticides contribute to ozone layer depletion.
Limit the use of private vehicles
One of the main causes of ozone layer depletion is the chemicals from the exhaust of vehicles. The vehicles on the roads should be limited which will result in less greenhouse gases. Also, stricter laws for pollutions should be applied in the future.
Employing environment-friendly products
In our homes, there are lots of household cleaning products available right now with harsh chemicals. These chemicals will find a way to the atmosphere. We can opt for use of natural environment-friendly cleaning products.
Stricter government actions on emissions
Nitrous oxide is also one of the contributors to ozone layer depletion. But it was not added in the previous Montreal protocol. There is a need of limiting and employing more stricter actions and laws from the government and they should be made to follow strictly. The major thing that happened to address the ozone layer depletion is known as the Montreal protocol. The ozone layer hole found in the Antarctica region is getting recovered because of these steps.
Montreal Protocol
In September 1987 about twenty-four countries took part in the Vienna convention. The agreements were done for phasing out the use of CFCs and other ozone layer-depleting substances (ODS).
This convention highlighted the two main points,
- issue of ozone layer depletion and
- helped to phase out the ozone layer depleting substances.
The Montreal protocol was applied on 25 August 1989 and the revisions were made in subsequent years.
- The countries that signed up for the Montreal protocol are made to phase out the use of CFCs. Climate projections indicate that the ozone layer will return to 1980 levels between 2050 and 2070. (Propane, Isobutane, etc).
- The Vienna conventions and Montreal protocol are now maintained by 196 countries. The co-operations and measures taken by each country made the success of Montreal protocols.
- The protocol was revised a number of times according to the present situations. In the early days, the ways of complete phasing out of chlorofluorocarbons were adopted.
- Later hydrofluorocarbons (HCFCs) were phased out. Though only a few countries phased out the use of HCFs but it’s expected to extend to more countries. Developed countries already acted and phased out the gases mostly according to the protocol.
- Lots of developing nations are also participating in the protocol. Keeping in mind the use of HCFs after phasing out the CFCs, the HCFCs were decided to phase out from 2016.
- The HCFs are having a high global warming potential so the use of HCFCs was limited and added to phase out from 2016.
Apart from phasing out the chemicals, there are also issues like there are not alternatives available right now to replace the ODS. Such as the halons that are very useful in fire suppression, there are no better alternatives available right now.
Some developing countries don’t have enough GDP to opt for better environmentally friendly options. They have to buy technologies from other countries and some countries couldn’t afford them. But the good thing is they are also slowly phasing out harmful gases.
Conclusion
Hence, we have got a basic idea all about ozone depletion potential, its causes, effects, etc.
