Open system, Closed system & Isolated system will be explored here. There are so many systems that are required to study in thermodynamics. A system means simply the collection of matter which needs to be studied in thermodynamics. It may be the water in a pipe or in an open container, or engine oil in the engine, or liquid in a heat exchanger, etc.
It is simply a collection of molecules or atoms or matters or other particles or space or region or quantity of matters which we are going to study in thermodynamics. We will explore these all!
Open System, Closed System & Isolated System
Now to understand the open system, closed system & isolated system, it is necessary to have basic knowledge of the thermodynamic system. Hence, we will learn about the thermodynamic system fast.
Basic of Thermodynamics
There are related terms that need to know in thermodynamics.
- System
- Boundary
- Surrounding
- Universe
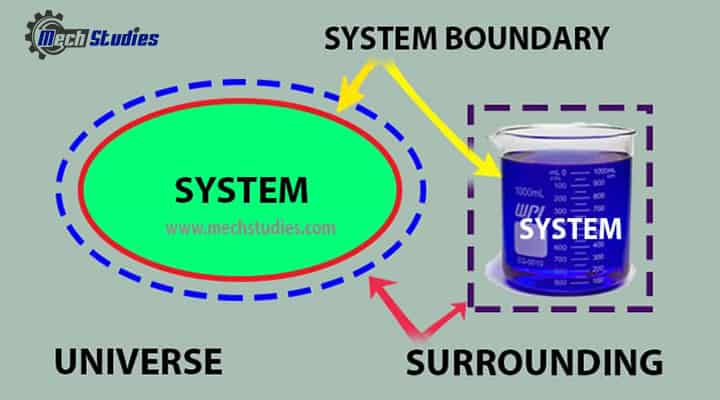
Everything external to the system is the surroundings, and the system is separated from the surroundings by the system boundaries. These boundaries may be either movable or fixed. Key terms are as follows:
System boundary universe
System
- Space or region or body where we measure the properties
- Space where the thermodynamic process happens.
- Space where the analysis is done.
Boundary
- Each system is bounded by a real or imaginary surface.
- This surface is known as a boundary or system boundary.
- It separates the system from the surroundings.
- The boundary can be movable or fixed.
- The shape and size of a boundary may change based on the system.
Surroundings
- Everything external to the system is surrounding.
- It means simply the outside of the system.
Universe
- It is a combination of system and surroundings.
- It can be written as, System + Surrounding = Universe.
Thermodynamic System
In thermodynamics, a system is that space or region or body where we measure the properties or where thermodynamics processes happened. That space or region or body is known as a system. We can define the thermodynamic system as the space, region, or quantity of matter which we study in thermodynamics.
- This quantity is measurable (in kg or lbs)
- In this system, we can measure the properties of thermodynamic parameters.
- In this system, the process is known as a thermodynamic process
Understanding with diagram
A simple diagram is illustrated to understand the thermodynamic system. Let us consider a gas balloon. What do you think, what is consisted inside the gas balloon?
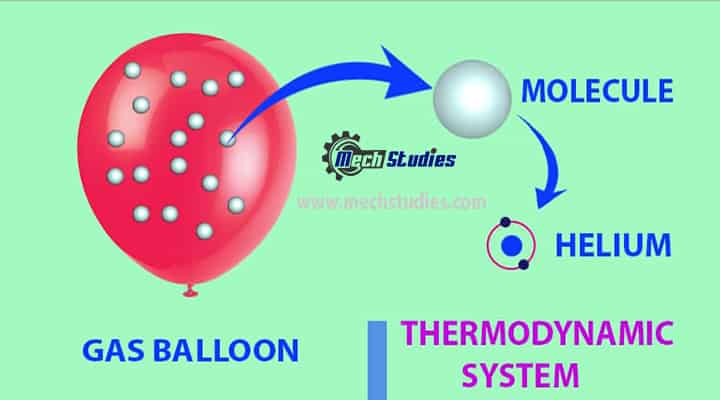
A system must always contain the same matter.
Other Examples
- A piston between intake and exhaust.
- Tea in a cup
- Water in the boiler
- Coffee in thermos flask etc.
Characteristics of Thermodynamic System
The thermodynamic system has the following characteristics:
- The Thermodynamic system must always contain the same matter.
- The thermodynamic system means a collection of matter or material or region in space to study thermodynamics.
- A thermodynamic system means the quantity of fixed matter with identity.
- The composition of the matter inside any system may be the same or maybe changed through various reactions.
- It contains matter and device(s) on a control surface.
- Its boundaries may be closed, may be open, may be movable, may be stationary, may be real or imaginary too.
- There may be a flow of energy in terms of heat transfer or work transfer.
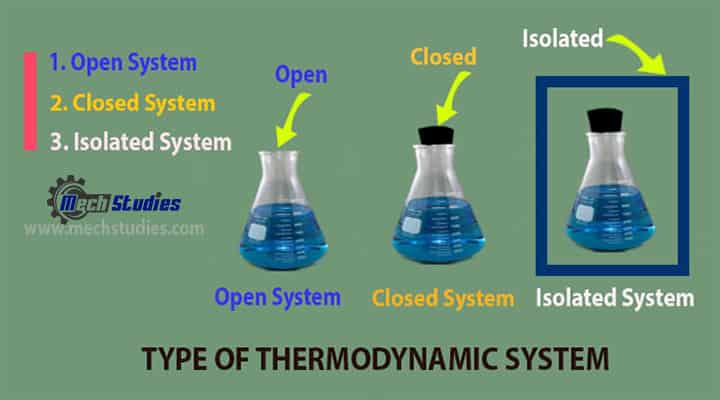
Types of Thermodynamic System
The exchange between the thermodynamic system and surroundings are based on two things,
- Exchange of matter or mass interaction.
- Energy exchange or energy interaction i.e., heat or work
Type of thermodynamics
- Open System
- Closed System
- Isolated systems:
These all types of thermodynamic system are elaborated, for understanding,
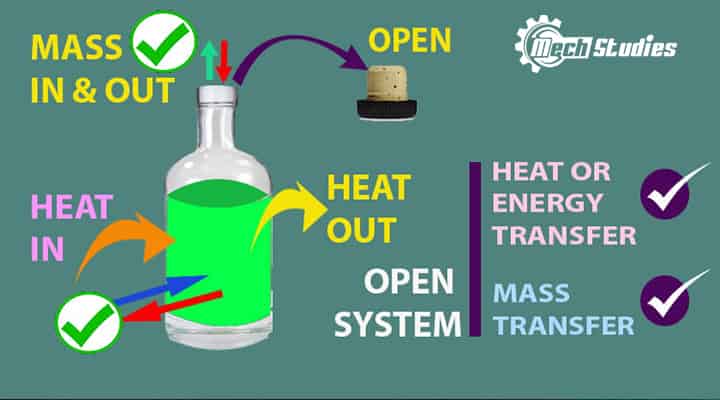
Open System
Open System Definition
An open system is a type of system where the transfer of mass, as well as energy, can be taken place across its boundary. It means in an open system:
- An open system is open to its surroundings
- Open system transfer mass across its boundary. Mass can transfer into the system or mass can go out from the system.
- The open system transfers energy across its boundary. Energy can transfer into the system or energy can go out from the system.
- Mass and energy interaction both are possible.
- The open system is also called as control volume.
Open System Example
Boiling Water
Description: Take some water in an open container and boil it. You will see the water is boiling and steam is coming out from the container and dispersed to the air.
Open system boiling water
Explanation
- There is some water in the container and during heating or boiling, you will see water vapour is coming out from the container or the system. Hence, mass transfer out from the system. So, we can say, it an example of mass interaction.
- Here, we are adding heat energy which converts water to vapour. Hence, the addition of heat means energy interaction into the system.
- Hence, energy and mass transfer happen between container and surrounding.
Compressor
Description: Let us consider a compressor. We all know, the compressor compresses the air in it and delivered compressed air.
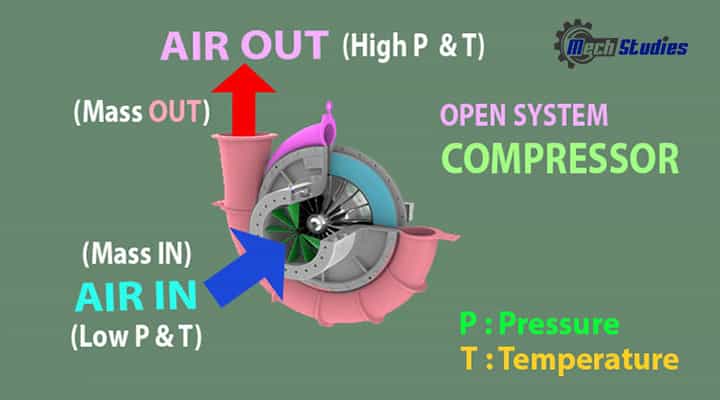
Explanation
- The compressor sucks normal air with normal temperature and pressure, delivers hot and pressurized air. Here, air comes into the compressor and go out from the compressor. SO, we can say, certain amount mas of air got interaction. Hence, it is an example of mass interaction.
- When the compressor rotates, there will be a change in energy. Here, we have seen the pressure and temperature is increased. How is it possible? In this case, rotation of the compressor impeller i.e. mechanical energy is transferred to the air and converts its mechanical energy into its kinetic energy as well as pressure energy.
- Hence, energy and mass transfer happen between system and surrounding.
- So this is the whole concept of an open system.
Engine
Engines, where we are putting oil and engine, produces power which is converted to mechanical energy.
Tea
If we pour tea in a cup, it will be acting as an open system.
Other examples are:
- Internal combustion engines
- Water pumps
- Steam engine
- Air compressors
- Boilers
- Turbines
Closed System
Define Closed System
A closed system is a type of system which is separated by a physical boundary from its surroundings. Close means the system is closed from its surroundings. In this system, energy can transfer across the system boundary in the form of Work or Heat but there will not be mass flow across the boundary.
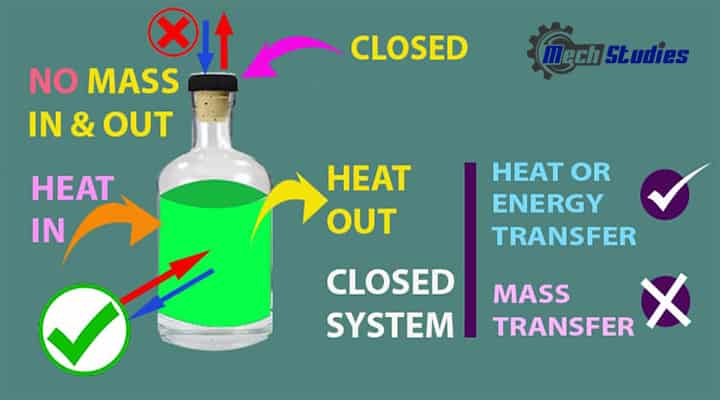
- The energy is transferred through the system boundary in a closed system.
- A closed system doesn’t transfer mass across its boundary.
- It’s a control mass approach
- In the case of the closed system, a safety valve needs to be considered to avert any kind of explosion.
Closed System Example
Piston – Cylinder
Description: Piston arrangement in a cylinder without valves is an example of a closed system. Keep some amount of fluid in the cylinder and close all the valves.
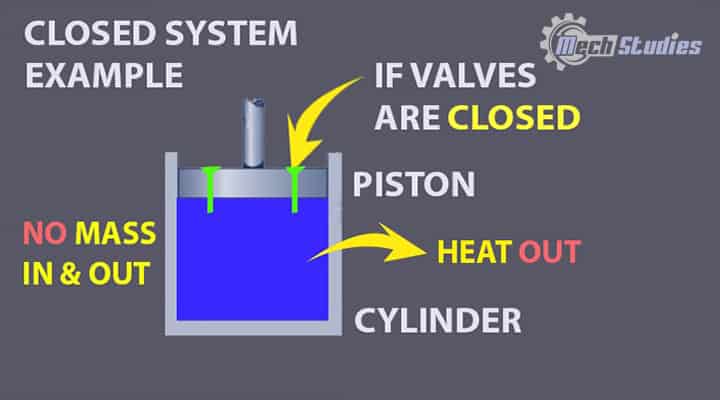
Explanation: Here, if we analyze, we see,
- the mass of fluid is fixed, and it cannot go out from the system or any mass cannot be added into the system, since, valves are closed.
- But if we put some energy into the piston, it will start to compress the fluid, and as a result, the temperature of the fluid will gradually increase, and that heat will be emitted outside.
- So, we added energy into the system and energy transferred too. Hence, there is energy transfer although there was no mas transfer.
Other examples are:
- Piston – cylinder arrangement without valves
- Sealed water bottle
- Gas-filled in a container
- Pressure cooker
Isolated system
Isolated System Definition
Isolation means totally separated from something. An isolated system is a type of system which is totally isolated from the surrounding so that there will not be any mass or energy transfer between the system and surrounding.
- An isolated system does not interact with the environment.
- Any changes in environmental conditions or surroundings don’t have an influence on the behavior of isolated systems.
- No mass and energy transfer across its boundary.
Isolated System Example
Thermos Flask
Description: The isolated system means there will not be any exchange of matter as well as energy across its boundary. It’s strange to explain.
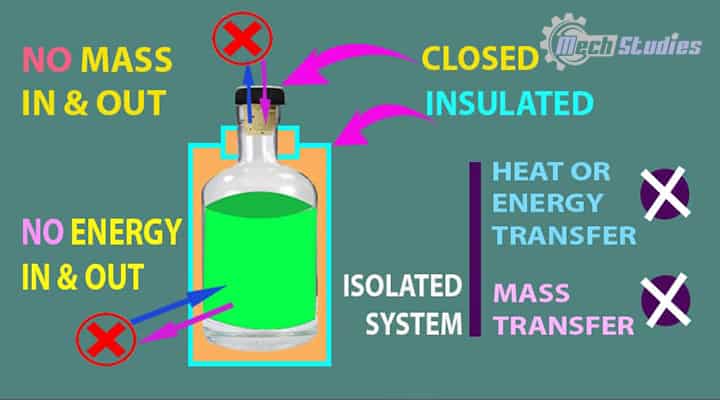
We all have a flask in our home. What do we do with it? We used to store hot water or hot tea or hot milk for a long time! How is it possible?

Explanation
Because this flask is well insulated, and it helps to prevent heat energy to go out and we get hot water. But have you observed the temperature of hot water after a long time is reduced and later on it will be same as normal temperature? Why? Because the insulation is not perfect. If the insulation is perfect, that means which will not allow any heat transfer across the boundary, then there will not be any energy transfer. Hence, there will not be any mass as well as energy transfer and this system will be known as a closed system.
Insulated Piston & Cylinder
Take some amount of gas in a cylinder, where the piston is already there. Close all the valves. If a gas is compressed or expanded in an insulated piston and cylinder arrangement, no mass or energy can transfer to the surroundings. This is a simple example.
Other examples are:
- Our universe
- Insulated water heater
Difference between Open and Closed system and Isolated System
The main differences between the Open System, Closed System, and Isolated System is, as follow:
| Sl No | Description | Open System | Closed System | Isolated System |
|---|---|---|---|---|
| 1 | Definition | It is the system, where mass or energy can be transferred. | It is the system where energy transfer happens but no mass transfer | It is the system where no mass & no energy transfer |
| 2 | Energy Transfer | Yes | Yes | No |
| 3 | Mass Transfer | Yes | No | No |
| 4 | Change in mass is possible or not | Changes | No change | No change |
| 5 | Boundary | Open | Closed | Closed |
| 6 | Reality | It exists | It exists | It is theoretical |
| 7 | Examples | Making tea in an open container | Keeping tea in a closed uninsulated container | Keeping tea in an insulated thermoflask. |
Example of a Few Common Thermodynamic System
We used to see a lot of thermodynamic systems, few of them are listed,
- Water in a pipe (Open system)
- Engine oil in the engine (Open system)
- Water Pump (Open system)
- The liquid in the heat exchanger (Open system)
- Boiler (open system)
- Turbine (Open system)
- Water is a closed pressure vessel (Closed system)
- Cooking in a pressure cooker (Closed system)
- Car Battery (Closed system)
- Piston cylinder with all valves closed (Closed system)
- A can of cold drinks (Closed system)
- Thermos Flask (Isolated system)
- Universe (Isolated system)
Is Universe an Isolated System?
We have already got an idea of an isolated system, and from that, we can say,
- The total energy of the universe is fixed.
- There is no change or transfer of energy with its surrounding.
- It automatically means that no mass transfer also undergoes.
- Hence, no mass & energy transfer means, the universe, is an isolated system.

Earth is a Closed System or Not!
If we analyze the energy system of the earth, we will see,
- Solar energy comes to the earth.
- Earth releases energy into the atmosphere.
- No mass is received or released by the earth.
- So, there will be energy transfer without any mass transfer.
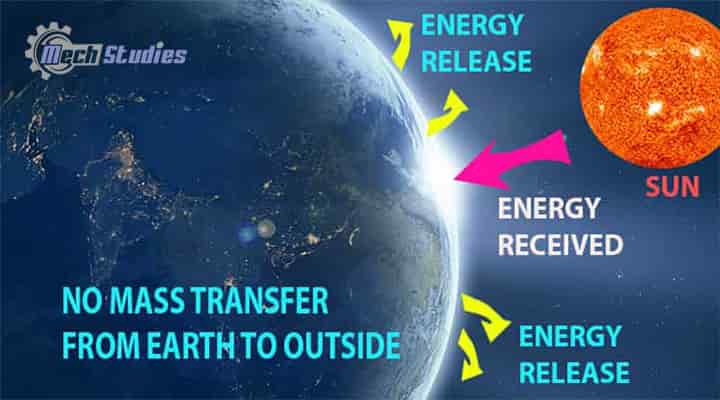
Hence, it is the same as the closed system.
Conclusion
So, we have learned the details of the open system, closed system, and isolated system along with various examples. Our doubt about the thermodynamic system is totally clear. Would you like to add some values to our articles, feel free to contact us!

