What is the third law of thermodynamics? Any idea! In this article, we will learn the basics of this law, definition, equation, example, etc. Let’s explore!
What is the Third Law of Thermodynamics?
In the field of engineering science, we know very little about the Third Law of thermodynamics and its concept. The Third Law of thermodynamics deals with the new concept; it includes the idea of temperature being absolute zero.
- We are known for the fact that the temperature surrounding us can get really hot or it can get really cold.
- But not everyone is friendly or familiar with the coldest temperature possible.
- But now with our new understanding of temperature and heat, it should make perfect sense.
In this article, we are going to learn more about the third law of thermodynamics and the crucial roles of absolute zero temperature as well as entropy. Let’s begin with this interesting topic then!
Absolute Zero in Third Law of Thermodynamics
- Think about the coldest place in the universe, it is the Boomerang Nebula in the Centaurs constellation at -272 °C or 1K. Since then scientists have been trying to achieve a lower temperature by different means and experiments. But even that didn’t result in something which is known as absolute zero.
- Since we can say that temperature is a measure of heat energy that is available, and heat energy is basically just a collection of kinetic energies of motion. Simply, when the temperature decreases, the internal energy of the system decreases, so the kinetic energy of each particle decreases.
- When the temperature becomes very cold, particles slow to a complete stop, and hence there is no more kinetic energy. As there is no more kinetic energy, we can say that there is no more temperature, and we will call this absolute zero. It is a part of Kelvin’s temperature scale where it corresponds to -273.14 °C or -459.67 °F
- An important thing to note here is that we may not be able to achieve absolute zero experimentally due to the second law of thermodynamics, but despite this, absolute zero is an important theoretical concept.
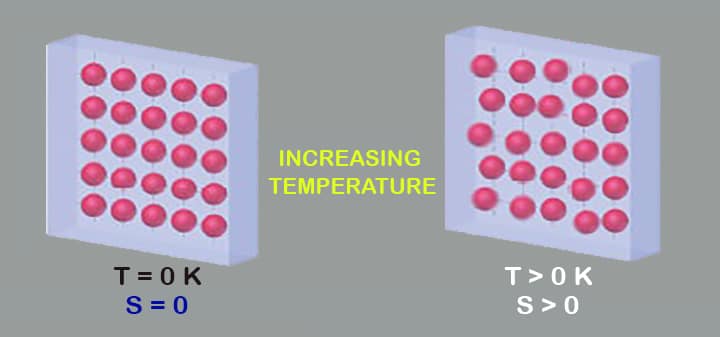
- At absolute zero, every possible substance is a solid, even some gases like hydrogen and helium. In another way, it encapsulates the Third Law of thermodynamics by saying the entropy of a perfect crystalline substance at absolute zero will be equal to zero.
Role of Entropy in 3rd Law of Thermodynamics
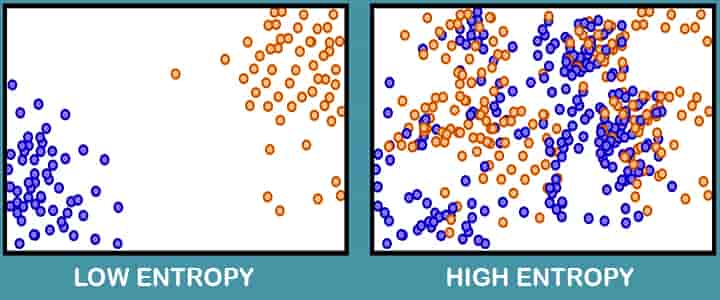
- Entropy can be defined as the thermal property of a substance that remains constant when the substance is expanded or compressed adiabatically in a cylinder.
- Entropy is the activity of a quantity of heat which shows the probability of conversion of that heat into useful mechanical work. The increase in entropy is very negligible when heat is added at a high temperature and is greater when heat addition is made at a lower temperature.
- Thus, for maximum entropy, there is minimum availability for conversion into work, and for minimum entropy; there is maximum availability for conversion into work.
- In thermodynamics theory, the term entropy plays a crucial role and leads to an important result which by other methods can be obtained arduously.
- It comes to an observation that all heat is equally beneficial for converting into useful work. It may be noted that the heat we supplied to a substance at a high temperature has a pronounced possibility of conversion into work than heat supplied at a lower temperature.
Fun fact about the word Entropy:
- The term entropy was created in the year 1865 by the German physicist Rudolf Clausius from the Greek word En = in + trope = a turning (point).
- The word reveals its relation to energy, and scientists believe that it was created to denote the form of energy that any energy eventually and inescapably turns into useless heat.
Characteristics of Entropy
- It increases when heat is supplied although it does not concern the fact whether the temperature changes or not.
- It can be denoted as heat which we can convert into work.
- In-All, the adiabatic frictionless process remains unchanged.
- Entropy is increasing parameters, thus entropy of the universe keeps increasing.
Entropy and Irreversibility
- Entropy is a thermodynamic entity, and it gives an idea about irreversibility. For reversible processes; change in entropy is a differential of the function of entropy, and the final conclusion of integration does not depend on the path of the activity or on how it is materialized, considering the fact that both the initial and final states are stable equilibrium states.
- Entropy is basically a closed adiabatic system, which remains similar in a reversible process, and it does increase during an irreversible event.
- A system and its surroundings create an isolated working system where the summation of the entropies of all bodies containing a reversible change remains the exact same, and it does increase during irreversible processes. Entropy can be used to discriminate between the two processes that are reversible and irreversible.
Statements of Scientists on Third Law of Thermodynamics
Planck’s statement on the third law of thermodynamics:
When the temperature of a substance is at absolute zero, the entropy of a perfect crystalline substance tends to be a constant. (Sometimes it can be taken to be zero)
Where,
S→0 at T→0
In the above equation, we selected S=0 at T=0 is called absolute.
- If S is contingent on a term say p (where we can assume “a” as an independent thermodynamic variable such as pressure or volume), then we can conjecture “a” to remain finite in the above equation.
- Planck’s formulation integrates the relationship between entropy, temperature, and the condition of absolute zero, but it does have an entry that states “pure crystalline substance.” This actually signifies its application to limited and specific substances. This statement excludes the entropy of a substance at T=0 which is not pure crystalline in nature, and thus it is not universally pertinent.
Einstein’s statement on the third law of thermodynamics:
When the temperature of a substance falls to absolute zero, the entropy of any substance remains finite.
S(T, x) → S0(x), |S0| < ∞ as T → 0, |x| < ∞
The limiting value of entropy at 0 temperatures depends on x, which is assumed to remain finite at T at 0.
Considering, the expression for the entropy change in a constant volume heating process
S = [ ∫ cv . cv/T . dT] 0 to T limit
It is easy to see that 1st equation presumes vanishing heat capacity
CV → 0 at T → 0
- This kind of similar result can be obtained for Cp considering the heating process with constant pressure.
- The statement proposed by Einstein who was investing about the entropy of quantum systems acting at low temperatures and concluding heat parameters should be at absolute zero; this also proved that S is finite at T=0.
- For validating Einstein’s statement about the third law of thermodynamics, Nernst along with his scientists conducted numerous experiments on the physical properties at low temperatures, which somehow confirmed Einstein’s statement. However, it should be noted that the thermodynamic systems become volatile at low temperatures, and thus they are likely to produce incorrect results, which are not so consistent with Einstein’s statement.
- Later, Debye corrected the quantum theory of heat capacity, which produced a convalescent match with Nernst’s results on the same subject.
- Whilst, Einstein’s statement on the third law of thermodynamics does validate on a certain, but this statement does not legitimize all-important thermal properties at T=0.
Nernst Theorem
The entropy change of a system in any reversible isothermal process tends to zero as the temperature of the process tends to absolute zero. It was originally known as the Nernst Heat theorem, but after that, it is called the Third Law of Thermodynamics.
S(T, x) − S(T, x + ∆x) → 0 as T → 0, |x| < ∞, |∆x| < ∞
The changing value in x is assumed to stay finite at T=0.
Assuming “smooth” differentiation, the Nernst theorem obviously implies that
(ds/dx) . T →0 as T→0
- We can consider the Nernst theorem valid in numerous cases, but we surely cannot say it is universal. Many times Einstein wrangled about Nernst’s statement accusing its derivation from the second law of thermodynamics.
- The main problem we are facing about this theorem is that the entropy which we considered cannot be an independent variable at T=0. In point of fact, if we are going to consider for a mixture of two or more substances (which might be two whole different substances or it can also be different isotopes of the same substances) can be escorted to the state o T=0, then there can be some precariousness in the positions of molecules since they represent a specific component of the mixture because to form a new microstate, we can swap the positions of the two different molecules.
- Considering a random variable let’s say “a” assuming a molar fraction of one of the components, we can culminate that presence of this precariousness should depend on the “a”.
- Nernst theorem gives a new direction regarding the state of the substance, contradicting the statement of Planck which only limited to pure crystalline substance.
- The Third law of thermodynamics is hence practically sort of related to the second law in terms of change in entropy but logically independent of the second law of thermodynamics.
Perpetual Motion Machine of Third Kind
- The law of thermodynamics is not truly related to theoretical validity, but it should also serve its purpose as applied significance and should have clear physical meaning. The term thermodynamic does have a very strong engineering element rooted in these principles. Scientists are still arguing about the fact that whether irreversibility in the real universe is only coupled with temporal boundary conditions imposed on the world or whether there are some other proceedings fundamental to irreversibility weaved into this subject.
- The fundamental imputations of the laws of thermodynamics are in fact correlated to heat engines (devices that are capable of converting heat into work). We can say that converting work into heat is an irreversible process since we can convert all work into heat, but we cannot convert all the heat into work.
- Now we can understand the third law of thermodynamics using a Perpetual motion machine of the third kind:
A Perpetual motion machine is basically a device that will work continuously without exchanging energy as heat or work from the surrounding. It means that it should not take any support from the surroundings and should work on its own to achieve the work done.
- Machines of the third kind are known to completely eradicate friction, thus they pursue a motion persistently due to inertia. Among the three kinds, machines that offer the third kind are the niftiest, and we can name them motion storage devices. They will never fabricate energy, or do more work than was we actually put into them, but low friction loss can help to make these machines a reality.
Let’s understand this machine with a simple example of a heat engine and a heat pump.
The above system consists of two devices which are a heat engine and a heat pump.
Let’s understand the working of the Heat engine and heat pump.
Heat Engine
In thermodynamic terms, a Heat Engine is a device that typically converts heat energy into useful mechanical work. This phenomenon is done by bringing a subject from a higher temperature to a lower temperature.
Heat Pump
Similarly in thermodynamics, a Heat Pump is a device that typically transfers heat from lower temperature to higher temperature using mechanical work provided by an external agent.
Now, in the above system, we use a heat engine and heat pump to understand the PMM of the third kind.
- Both the devices are connected to each other by the mechanical work provided by the heat engine. The Heat engine draws the higher temperature heat energy from the pump and produces some mechanical work which then supplied to the heat pump. The Heat pump then draws lower temperature energy and converts it into higher-temperature energy by using mechanical work produced by the heat engine.
- Now by studying these systems we can come to the conclusion that it does not violate the 1st as well as 2nd law of thermodynamics since it follows the statements.
- Although it does not violate the laws this kind of machine is only possible theoretically, it cannot be possible practically since there is friction present in the system.
Conclusion
These statements as well as laws are considered as pillars of thermodynamics since all the different concepts are derived from these laws. Theories of these laws are utilized to make them applicable in today’s life. While studying the Third Law of thermodynamics, we concluded that a frictionless device is not possible practically, although for achieving maximum efficiency we can focus on the entropy changes.
