Intensive properties and extensive properties are the most interesting properties which are explained in the simplest way. There are certain characteristics to understand the physical conditions in every system. These are mass, pressure, temperature, volume, density, color, boiling point, etc. and these all are known as properties of the system. Let’s explore the concept!
Intensive Properties and Extensive Properties – Definition
Intensive and Extensive Properties Concept
At the end of a long day, nothing is more soothing than a good chocolate cake. The refreshing nature of a chocolate cake won’t be appreciated if you are hungry, but if you are feeling better, a brownie won’t help you. In spite of its inseparability from pastry, a cake can provide enough calories to satisfy your appetite. Furthermore, certain characteristics of matter vary with sample size, whereas others remain the same. In this way, a matter’s qualities can be classified into two categories: those that are extensive and those that are intensive.
There are lots of Common examples of these properties which include mass, volume, energy, temperature, etc. A system’s extensive properties can be divided into separate parts for the sake of convenience; the total value of an extensive property is the sum of the values for the individual parts.
Intensive and Extensive Properties Basics
Intensive properties and extensive properties! Let’s take any two properties from the above, mass, and temperature to explore the concept of these two properties!
- Now, if we increase the quantity of matter, the mass will increase but the temperature will not be changed.
- If we increase the size of the matter, the mass will increase but the temperature will remain the same.
From this, we understand that few properties depend on the size or quantity of matter and few properties don’t. Properties of the system are classified into two categories based on how properties are changed with respect to change in system size or quantity of matters. These are, namely,
- Intensive properties
- Extensive properties.
Types of properties Intensive properties Extensive properties
Intensive Properties Definition
What are the Intensive Properties of Matter? An intensive property is defined as the properties which don’t depend on the size or the amount of the substance present in a system. Temperature is a property that doesn’t depend on the size or the amount of matter. Hence, these properties are known as intensive properties.

Extensive Properties Definition
What are the Extensive Properties of Matter? The extensive property is different from the intensive property, and it depends on the amount of matter or the size of the system. The mass of any substance depends on the size or quantity of matter. Say, 10 kg of rice!
If 1 kg of rice is added, the mass of rice will increase and it will become 11 kg. Hence, this property depends on the quantity and it is a simply extensive property.

Intensive Properties and Extensive Examples, Symbols
Let’s try to understand the examples of intensive and expensive properties by taking 5 liters of water in a bucket.
Intensive Properties Examples
Here,
- the volume of water is 5 liters
- we know the density of water
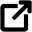 is 1000 kg/m3
is 1000 kg/m3 - mass of water = volume x density = 5 kg.
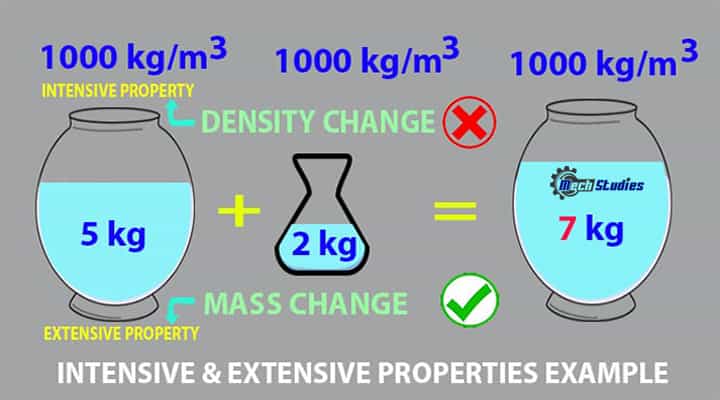
Now, if we add 2 kg water to the bucket, the new mass will be (5 + 2) = 7 kg. If you measure the density of water now, do you think the density of water will be changed? No, it will not change! Hence, density doesn’t change with respect to quantity and hence it is an intensive property.
Characteristics of Intensive Properties
The intensive property of a system has the following characteristics:
- This property is not changed with decreasing or increasing the amount of the substance.
- It is totally independent of the amount of matter or size.
- No change of the size, if these properties change.
Symbols of Intensive Properties
Intensive properties are symbolized by the lower case (small letters) such as:
- Specific volume: v
- specific kinetic energy: ke
- Specific internal energy: u
- Specific enthalpy: h
- Molar specific volume,
- Molar specific energy,
- Molar specific kinetic energy
- Molar specific potential energy respectively.
Extensive Properties Examples
W have added 2 kg of water in the bucket, the new mass is (5 + 2) = 7 kg. So, mass is changed with size or quantity. Hence, mass is an extensive property example.
Characteristics of Extensive Properties
The extensive property of a system has the following characteristics:
- An extensive property is a physical quantity that depends on the amount of matter or substance.
- This property is totally dependent on the amount of matter or size.
- Change of these properties happens with a change in the size of the system.
Symbols of Extensive Properties
Extensive properties are normally symbolized by the upper case (capital letters) such as:
- Volume: V
- Kinetic energy: KE
- Potential energy: PE
Exceptions of Symbol for Intensive & Extensive Property
Temperature is an intensive property, but the symbol is the capital letter ‘T’. Mass is an extensive property, but the symbol is the small letter ‘m’. The number of moles is extensive property but the symbol is small i.e. mol’. These symbols are traditionally used for a long time and continue the use.
Differences Between Intensive and Extensive Properties
There are a few differences between Intensive and Extensive properties:
| Sl. No. | Description | Intensive Properties | Extensive Properties |
| 1 | Basic | It is not related to the amount of matter or substance | It is related to the amount of matter or substance |
| 2 | Whether it is dependent on amount of matter? | Independent | Dependent |
| 3 | Whether size is changed with the change this properties? | No change | Yes, Changed |
| 4 | Whether it can be easily identified? | Yes, easily identified. | Not easily, since it depends on matters |
| 5 | Whether it can be Computed? | Difficult | Easily computed |
| 6 | Examples | Pressure | Volume |
Note: These two properties can be changed, measured, or observed without changing the identity of matter.
How to Identify Intensive & Extensive Property?
Take some water in a bowl, hence it is a system. Water has many properties like mass, volume, density, temperature, etc. and the same can be measured easily. Now, if we divide this water into two containers means two systems, and measure all the above values, we will see some differences.
- If the value is not changed then it is an intensive property.
- And if the value of any property is changed then it is an extensive property
In the above case, if we observe, we will see that mass and volume is changed due to the change in the system. Hence, volume and mass both are extensive properties. However, density and temperature will be the same as earlier with system change and these are intensive properties.
How to Remember Intensive & Extensive Property?
Intensive properties are independent of size or quantity, simply Independent. Hence, we can conclude, INtensive means INdependent and easy to remember. Extensive is simply the opposite.
Special Note: The ratio of two extensive properties is not scale-variant, and it is, therefore, an intensive property.
For example,
- Density = Mass (kg)/Volume
- Density = Extensive property/Extensive property
- Density = Intensive property (kg/m3)
Hence, the density of water or any liquid is an intensive property.
Other examples, Specific Volume
- Specific Volume = Volume (m3)/Unit mass (kg)
- Specific Volume = Extensive property/Extensive property
- Specific Volume = Intensive property (m3/kg)
Another example,
- Specific enthalpy = enthalpy (J)/Unit mass (kg)
- Specific enthalpy = Extensive property/Extensive property
- Specific enthalpy = Intensive property (J/kg)
Check the property of specific entropy,
- Specific enthalpy = entropy (J/K)/Unit mass (kg)
- Specific enthalpy = Extensive property/Extensive property
- Specific enthalpy = Intensive property (J/kg-K)
Now, let’s check the property of specific heat capacity,
- Specific heat capacity = heat capacity (J/K)/Unit mass (kg)
- Specific heat capacity = Extensive property/Extensive property
- Specific heat capacity = Intensive property (J/kg-K)
Intensive Property and Extensive Property List
Intensive properties List
- specific internal energy, u
- temperature, T
- Specific enthalpy (h)
- Amount of substance (mol)
- concentration, c
- density, ρ
- Energy (E)
- Entropy (S)
- Gibbs energy (G)
- viscosity
- Helmholtz energy (A) etc.
- chemical potential, μ
- melting point
- Hardness
- Hardness
- Magnetization
- Luster
- Magnetic field
- specific rotation, α
- Malleability
- magnetic permeability, μ
- Ductility
- Solubility
- specific volume, v
- boiling point
- Elasticity
- pressure, p
- Refractive Index
- molality, m or b
- Specific conductance (or electrical conductivity)
- specific heat capacity, cp
- standard reduction potential, E°
- surface tension
- thermal conductivity
- Colour
Mathematical Example of Intensive Property
1 kg of water boils at 100 deg. C, what will be the boiling temperature of 5kg of water?
Explanation for Intensive Property
Melting point or freezing point or boiling point etc. are intensive properties and we know, intensive property means it doesn’t change with the amount of object. So, the boiling point doesn’t depend on the amount of water. Hence, the boiling point will be 100 deg. C.
Extensive properties List
- Mass (m)
- Volume (V)
- Length (L)
- Energy, E
- Internal Energy, U
- Heat capacity (Cp)
- Surface area
- State of Matter
- Weight
- Number of molecules
- Electrical charge
Mathematical Example for Extensive Property
1 kg of water is added to 5 kg of water, what will be the total mass of water?
Explanation for Extensive Property
Mass is an extensive property, which means it depends on the mass. Hence, 1kg of water and 5 kg of water together give 6 kg of water.
Exercise on Intensive & Extensive Property
Question: Identifying Intensive and Extensive Properties from the below list with justification,
- Mass
- Density
Solution
Mass: Extensive Property
I have 2 kg sugar in a big container, so, the mass of sugar is 2 kg. Now, if we make it half and keep it into two different small containers, then each container consists of 1 kg sugar.
- Mass of big container = 2 kg
- Mass of big container = 1 kg
Hence, mass is changed here, so, it is an extensive property.
Density: Intensive Property
Take a big bucket of oil and measure the density. Now, take this oil and fill it into a few small buckets.
- The density of oil in a big bucket = 850 kg/m3
- The density of oil in a small bucket = 850 kg/m3
The density of oil in the big bucket = the density of oil in the small buckets. Hence, no change in density, now, no change means it is an intensive property.
Density, ρ
- ρ = mass/volume
- ρ = mass (extensive property)/volume (extensive property)
- ρ = intensive property
Mathematics Explanation of Extensive Properties
There are three variables P, V, and T which are called independent variables that affect the extensive properties of a single or pure substance. It is only the two independent variables that will determine the extensive properties of the system if the n is kept constant.
It depends on the moles n of the constituents A, B, C, etc., as well as the two independent variables that determine the extensive properties of a solution of two or more substances. In addition to the two independent variables, the intensive properties of the species will depend on their concentration.
Suppose PV = nRT has two comprehensive variables n and V and two intensive variables P and T. It is, however, possible to obtain three intensive variables, c, P, and T, by combining the two extensive variables of the ideal gas equation.
P= cRT
Frequently Asked Questions for Intensive Properties & Extensive Properties
What are the extensive properties?
It is a property that is related to how much material is present in a sample as well as being known as extensive quantities. There is an additive relationship between these properties for subsystems. Whenever the value of the property of a system equals the sum of the values for its components, the property is described as extensive. There are many properties that are extensive, including volume, energy, and mass.
It follows that as a system’s mass rises, its weight increases as well. In the same way, the volume of a substance increases as its mass increases. The heat capacity of a system increases as its mass increases. It depends on the mass of a system and how much energy it stores.
Their properties can change depending on the conditions, so they cannot be used for identifying samples of matter. It is a physical property that has an extensive range of properties.
Despite their usefulness in describing, extensive properties aren’t helpful in identifying samples since they can change depending on size or conditions.
What are five extensive properties?
There are five extensive properties; these are the part of the physical extensive properties.
They are as follows:
● Mass
● Volume
● Size
● Length
● Weight
What are the other five extensive properties?
There are other extensive properties; these are the part of the chemical properties
They are as follows:
● Enthalpy
● Energy
● Entropy
● Gibbs energy
● Heat Capacity
Can you think of a property of matter that is extensive?
Several properties of matter could be classified as extensive properties, and their value changes as a matter’s size or quantity changes. These properties are called extensive properties of matter.
For instance, two crates made from the same material but with different weights will also have different properties.
Is freezing considered to be intensive or as extensive?
The property of freezing is considered to be intensive. As the amount of a substance changes, the freezing point does not change. Therefore, water’s freezing point is constant regardless of quantity.
Is water considered to be intensive or extensive?
In order for the water to begin boiling, the temperature must reach 100 degrees Celsius. Thus, the boiling point is a property that is intensive. As with boiling point, melting point is an intensive property as well.
Is viscosity intensive or extensive?
Viscosity is determined by the resistance of liquids to flow. Consequently, viscosity is an intensive property since it does not change with matter amount. As a result, viscosity occupies little space.
Is malleability intensive or extensive?
A malleable material is one that can easily be hammered or rolled into a thin sheet. Composition affects how malleable an object is, so it is an intensive property.
In what ways can Extensive Properties be distinguished from Intensive Properties?
When you are combining two samples of the same type of matter, it is easy to determine whether a property is intensive or extensive. There is no difference in the intensity of a property regardless of how big or small the sample is.
The density, temperature, and hardness of a small amount of matter are the same as those of a large amount of the same substance. A property that is extensive, on the other hand, is additive. A sample should be twice as large as it is so that an extensive property is doubled. As a result, doubling the sample would make it twice as large, twice as long, etc.
What are the specific properties of extensive and intensive?
Specific properties are special types of intensive properties based on the ratio between them. Volume and mass, for instance, are both extensive properties. They are both intensive properties and specific properties, and their ratio is density. Additionally, enthalpy, heat capacity, molar volume, and specific volume of a substance are important factors to consider.
Is Heat extensive or intensive?
The heat is an extensive property. A large quantity of matter will therefore have a proportionally large heat capacity, since heat capacity can be scaled up as a function of how much matter is present.
Is temperature extensive or intensive property?
Temperature is an intensive property. It is true that a drop of coffee has the same temperature as a cup of coffee, but thermal energy depends on its amount, so it is a property of considerable scope
Is density extensive or intensive?
Since density is independent of the amount of material, it is an intensive property of matter. As a measure of mass, density shows how much mass a substance has in a given quantity. Depending on the intrinsic nature of a substance, density is an intensive property. As a result of the narrow range of densities between samples, density is an intensive property. Densities remained the same regardless of initial mass.
Is flammability intensive or extensive properties?
Flammability is an intensive property because it is an effective method for producing oxygen. Having a lot of hydrogen may make it more noticeable than having a little, but the fact that it will burn remains the same.
Which are intensive properties?
Matter that has intensive properties is unaffected by its size or quantity. Size, density, color, melting and boiling points, and other properties are not affected by size or quantity. Water’s density will remain constant regardless of whether it is 1 liter or 2000 liters.
It is possible to determine whether an electric conductivity is intensive or extensive from water’s electrical conductivity based on its consistency regardless of volume.
What are 5 intensive properties?
Temperature, density, viscosity, color, melting point, etc., will not change based on the size or quantity of matter. Due to its intensive property, the density of 1 liter of water or 100 liters of water will remain the same.
What are 3 intensive properties?
Pressure, odor, flammability are three intensive properties.
How would you define an extensive physical property?
An extensive property is volume, energy, and mass, among others. The property of capability or extensiveness is defined as a property whose value depends on the quantity of a substance
Describe a property of matter that is intensive: what is it?
The significance of an intensive property of matter is not affected by its size or quantity. Intensive properties include temperatures, density, color, melting and boiling points, etc., as they won’t change as matter size or quantity changes.
The intensity of a substance does not depend on its amount. The intensity of a substance can be measured by its color, taste, or melting point. Depending on how much matter is present, extensive properties can vary. Mass, volume, and length are three proper examples of extensive properties.
What are the difference between Extensive & intensive properties?
Physical and chemical properties are inherent in all matter. The properties of some compounds may be unique, while those of others may be similar to those of others. It is possible to observe physical properties of matter without changing the chemical composition of the matter. Intensive properties and extensive properties are two types of physical properties.
Mass properties are not affected by the amount of matter; they are known as intensive properties. The amount of matter determines the extent of a physical property. Extensive properties have a larger amount of matter.
Conclusion
Hence, we have learned the basics of intensive properties and extensive properties along with a lot of examples.


Thank you for some other fantastic article. Where else could anybody get that type of info in such a perfect method of writing? I’ve a presentation subsequent week, and I am on the search for such info.