What is a Pure Substance? Any idea! In this article, we will learn all about pure substances, definition, various compounds, the difference between pure substance vs mixtures, examples, diagram etc.
Let’s explore!
What is Pure Substance? Definition & Examples
Let’s try to understand the basics of pure substance and its definition.
Pure Substance Basics
When we look around us we see things that are all made up of matter. From street lamp to vehicles, from clothes to food, the air we breathe is also made up of matter.
- We many times see advertisements of Pure GHEE, what is this pure concept?
- Are all substances are pure? Whether milk, juice, mineral water are they pure?
Here the meaning of pure is the product is free of adulteration. But in scientific terms that is not a meaning of pure substance.
Pure Substance Definition
For example, milk is made of water, fat, proteins etc. and it is actually a mixture. Then what is a pure substance?
Basically, pure substances are those, when they will be disintegrated into small parts, all those parts will have the same composition.
Pure Substance Examples
We will discuss all characteristics, basics of pure substances, however, a few examples are illustrated, for a concept
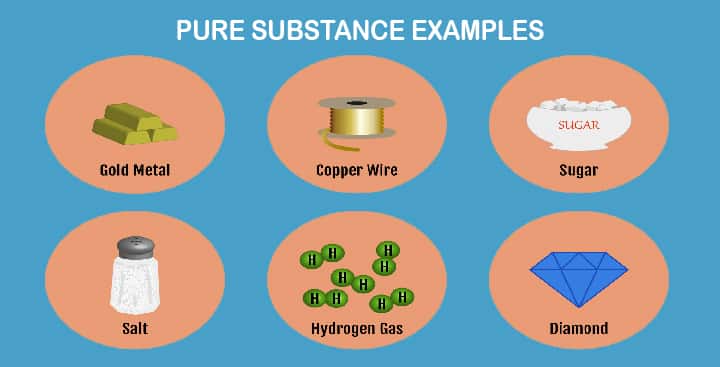
- gold,
- sugar,
- carbon,
- copper
- aluminium,
- silver,
- boron,
- H2O,
- salt,
- diamond, etc.
Matters vs Pure Substance vs Mixtures & Examples
Let’s try to understand the state of matters first before understanding the types of matters or pure substance.
States of Matter
This matter is made of particles that are very small in size we call them atoms. Matter mainly exist in solid, liquid and gas state. What makes something solid, liquid or gas is dependent on the internal structure of the atom.
These states of matter are inter-convertible which means they can be changed by the use of temperature and pressure.
- Solid: If atoms are closely bind to each other then it is called solid and as they are closely spaced the forces of attraction between them are maximum. The whole structure is very much arranged in orderly manner. Molecules of solid don’t move relative to the position of other molecule.
- Liquid: While in case liquid they are loosely bind, due to this reason interatomic forces are not so strong. In liquid one layer slips over another and hence they are fluid.
- Gases: In Gases they very loose and away from each other. Weakest molecular bond exists in them. They are moving just randomly and also collide with each other. These molecules have high energy so it must be released in order to freeze them.
Types of Matters
The matter is made of pure substances and Mixtures, which means the matter is classified,
- Pure substances
- Mixtures
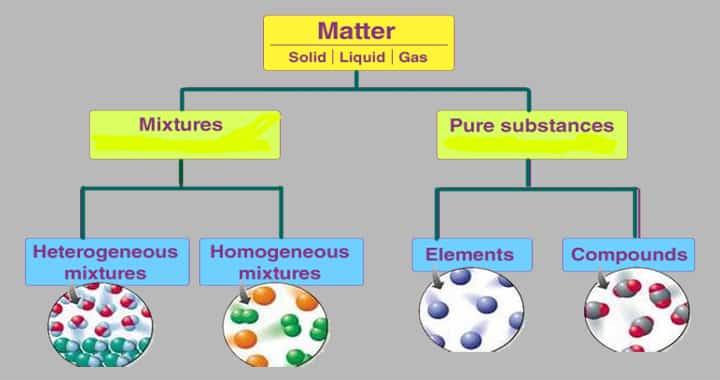
These pure substances are again subdivided into,
- Compounds and
- Elements.
Compounds are of different types and we will go through them in the later part of this article. Whilst mixtures also have many types which are also explored in this article.
Any substance will be called pure as long as its composition remains the same throughout. To know this we need to dive in-depth to know many concepts.
Let’s try to understand each type of pure substance.
Description of Pure Substance Types
Atoms are tiny particles that makeup matter when bonded together. Basically, substances are classified as Element, Compound.
Element
What is Element of Pure Substance?
Is an element a pure substance? Antoine Lavoisier was the first one to come up with the definition of the element back in the 1700s. According to him, it is the basic simplest of all substances that can’t go through a further breakdown, even if a chemical reaction is used.
So basically element has only one specific type of atom. Elements can be found in 3 forms- metal, non-metal and metalloid. Known elements to us are about 100 of which 92are naturally occurring and the rest are manmade. Generally, the majority of them are solid.
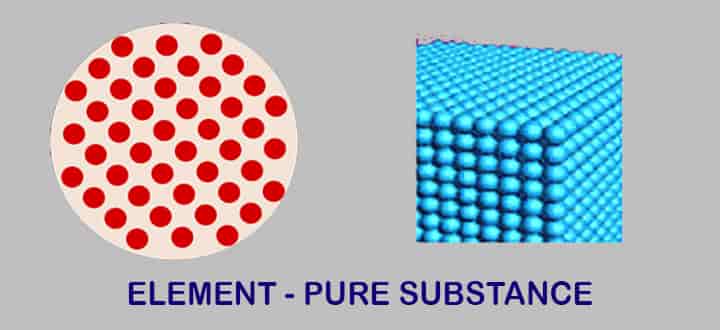
Types of elements
Metals
Elements that are electropositive in nature lies on the left side of the periodic table. They have properties like lustre, sonorous, good conductivity of heat and electricity etc.
Example
- gold,
- silver,
- aluminium, etc.
Non-metals
Elements that are electronegative in nature lie on the right side of the periodic table. They have low heat and electrical conductivity due to their atomic structure.
Examples
- carbon,
- boron, etc.
Metalloids
They are also known as semi metals because they have properties of both metals and non-metals.
Example
- germanium.
Compounds
What is a compound?
Compounds are formed when two or more elements are combined chemically with each other in a fixed specific proportion.
Chemically combined is the basic tenet. Water is a hydrogen-oxygen molecule bonded together as a compound.
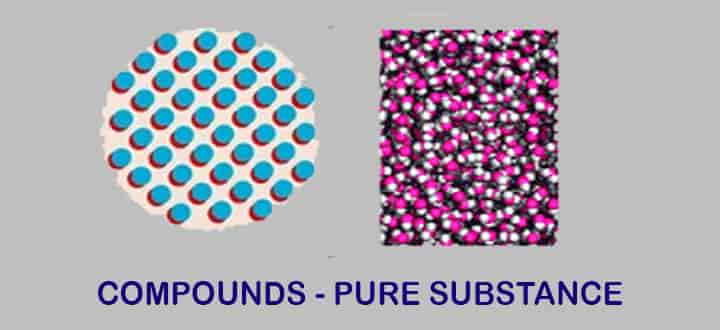
Types of compounds
- Based on elements present
Oxides – When a compound contains one or more oxygen atoms. Generally, metals tend to form oxides fast. (MgO) magnesium oxide or ferrous oxide. (Rust)
Hydrides – When a compound contains one or more hydrogen atoms.
Halides – When a compound contains one or more halogen atoms.
Organic – When the compound contains carbon and hydrogen. Generally, all that is of plant and animal origin is considered organic. Proteins, Carbohydrates are some examples.
Inorganic – When a compound doesn’t contain carbon then it’s inorganic.
- Based on bonding between compound
Ionic compounds – these are compounds that are formed due to strong attractive forces among oppositely charged ions. NaCl is the best example of an Ionic compound.
Molecular compounds – these are compounds that are formed by sharing of electrons. Depending on the number of electrons shared covalent bonds are called single or double covalent bonds.
Ex water (H2O), Methane (CH4)
- Based on reactivity and types of chemical reaction
Acids – these are the compounds that produce H+ ions when they are being dissolved in water to create an aqueous solution. They always have a pH value less than 7.
Ex (HCl) Hydrochloric acid, (HNO3) Nitric acid
Bases – these are compounds that accept these positive ions. They always have a pH value greater than 7.
Ex (NaOH) Sodium Hydroxide
Neutral – these compounds have pH=7
Ex water (H2O)
Differences between Elements and Compounds
| Elements | Compounds |
| They cannot be disintegrated into simple substances. | They can be disintegrated into simpler substances that are elements by using chemical reactions. |
| Ex gold, silver | Ex water, methane |
Is Oxygen a Compound?
Atom is a single particle in itself. But a Molecule is formed when two atoms join together.
Ex Oxygen (O2) is a molecule because it consists of 2 atoms. While Nitrogen (N) is an atom. So it becomes confusing, we just learnt compounds are made of two or more elements.
So oxygen is a compound? No. It is an element only because it is made of a single element.
It will become very easy to understand what pure substance is if we learn about mixtures first. Because the mixture is something we see abundantly in surrounding so it will become easy to differentiate it.
What are Mixtures? Types and Examples
Mixtures
The mixture is made of a variety of elements, compounds and other substances which are not chemically combined.
It is made of a mixture of different kinds of matter which in itself is in pure form.
Salty water is a mixture of water and sodium chloride (NaCl).
But this sodium chloride can be removed from water by use of evaporation but sodium chloride in itself cannot be separated into two quantities – sodium or chloride.
Let’s see the types of mixtures,
- Homogeneous mixture
- Heterogeneous mixture
Homogeneous Mixture
The mixture is said to be homogeneous when it has the same composition throughout. It doesn’t get separated into phases when left unstirred. Any homogenous mixture becomes a solution when it has solute and solvent.

Example:
An alloy is a homogeneous mixture in which two or more elements are combined of which one must be a metal.
Heterogeneous mixture
Such type’s mixture doesn’t have a fixed composition. They get separated into phases when they are left unstirred. The separation process is very simple and can be done by ordinary means.
Example:
Blood, Oil and Water.
Separation of Mixtures
By using evaporation
By this solute is recovered in powdered form as the solvent evaporates.
By using filtration
The residue is caught by the filter
By using centrifugation
Sometimes very small solid particles are there and filters cannot be used. In this denser particles settle at the bottom while lighter ones stay at the top.
By using distillation
This process is used where both miscible liquids have different boiling points. Fractional distillation is used to derive products from crude oil.
Solution
It is a homogeneous mixture made of two or more substances. Basically, homogeneity lies at the core of the solution, it has to be the same throughout. It may be solid, liquid or gas.
Ex lemonade and soda water are common examples. It comprises of a solute and a solvent, solvent is the component which is available in larger quantity and solute is the in small quantity.
The solute is dissolved into the solvent. The solute particle cannot be removed when the solution is left unstirred, they don’t settle at the bottom nor can they be removed by the process of filtration.
Depending on the amount on quantity of solute, it can be dilute or saturated. When it happens that no additional solute can be dissolved then it can be said that solution is saturated.
Ex If salt is mixed with water then it is a solution.
Suspension
A heterogeneous mixture in which solute particles tend to suspend in solution rather than getting dissolved. These suspended particles can be seen by the eye and they can distort the beam of light passing through them. When left unstirred, the solute particles settle down and can be removed by the filtration method.
Ex Mixture of soil and water, a mixture of flour and water.
Colloidal solution
If we go by appearance it looks like a homogeneous mixture but actually, it is a heterogeneous mixture. The solute keeps itself dispersed but does not settle to the bottom.
They are too small to settle down. The substance which gets dispersed is called the dispersed phase while the substance in which it is dispersed is called dispersion medium. When a beam of light is passed through it then it gets dispersed in all directions and this effect is known as the Tyndall effect .
.
Example: Fog is colloid or sun rays passing through the shade of a tree then you can see Tyndall effect. Milk is also a colloid of liquid butterfat globules which are dispersed in water.
Applications
Suspension – mainly used in medical sciences. Barium sulphate solution is used as a radiocontrast agent in x-ray imaging.
Colloids – mainly used in the medical field, used to regulate colloidal osmotic pressure, which is a pressure applied by proteins in the blood to pull water into the vascular system.
Properties of Pure substance
- They have definite physical and chemical properties and they don’t keep changing from sample to sample.
- They are made of just one type of particle.
- Elements and Compound are its 2 types.
- Physical process such cutting, breaking or melting doesn’t change their properties.
- If they go throw chemical change then only they change properties.
Differences Between Pure Substances and Mixtures
There are many differences between pure substances and mixtures, as follows:
| Pure Substances | Mixtures |
| Based on the definition, the pure substance cannot be separated into new substances. If a pure substance is broken down, the smallest piece will be also same characteristics. | The mixture can be separated and get the substances with the help of different methods |
| These substances are made of one particular element | The mixture means as the name suggests, a combination of two or more substances. |
| The physical properties and chemical properties of a pure substance is constant | The physical properties and chemical properties of a mixture are variable. |
Phase and P-v diagram of Pure Substance
Let’s see the Phase and P-v diagram of Pure Substance,
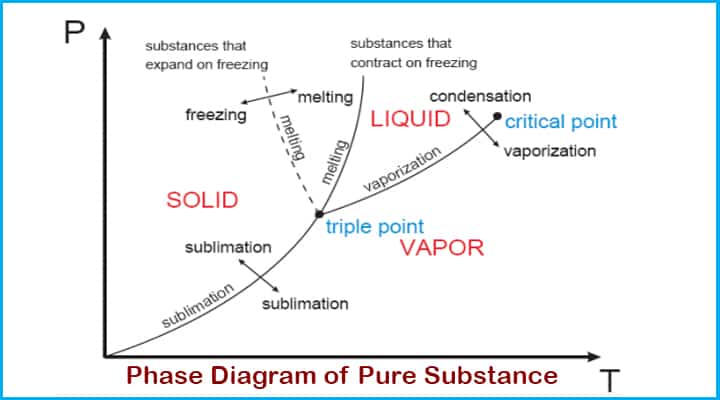
Phase Diagram of Pure Substance
Let’s see a sample phase diagram of pure substance,
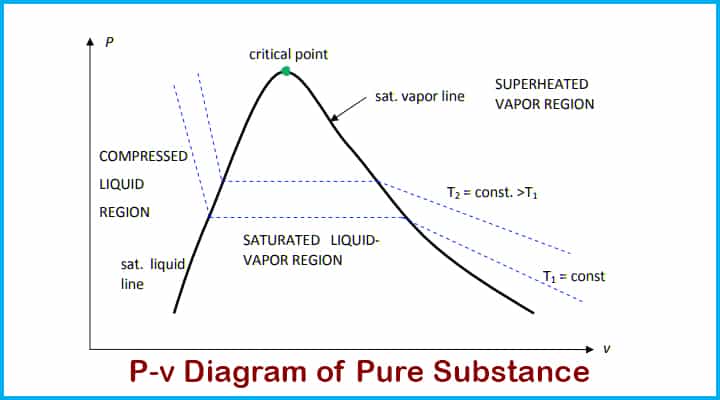
P-v diagram of Pure Substance
Let’s also see P-v diagram of pure substance,
Effect of Temperature and Pressure on Pure Substance
As per the application of pressure and temperature the pure substance can go through a phase change.
To understand this let’s go through an example.
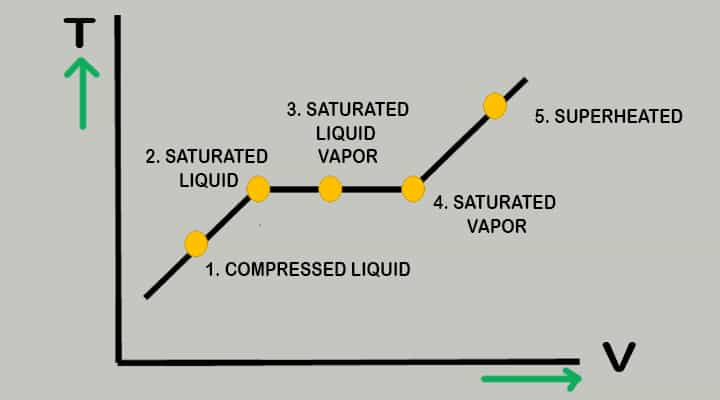
Water at room temperature is kept in a piston-cylinder device. Its temperature is 27 degrees Celsius. The water is in the liquid phase and it is called a compressed liquid or Subcooled liquid that means it is not going to vaporise now.
If we continue to heat this water till 40 degrees then its specific volume will increase and this will be resulting in process of slightly pushing that piston upward. If we continue adding heat up to 100 degrees then water has reached its saturated liquid point, because we increase temperature water will start evaporating and producing vapours. This specific point where water will start to vaporise is known as a saturated liquid.
If the continuous addition of heat is continued the vapours will form on large scale. The only thing which will change will be the specific volume. This will continue until the whole block is filled with vapour. This point is called saturated vapour.
There is one state between saturated vapour and saturated liquid where these two phases coexist is called saturated liquid vapour mixture.
After the saturated vapour stage if the continuous addition of heat is continued then it will turn into superheated vapour.
The saturation temperature is the one at which the phase is changed of pure substance.
The pressure at which pure substance changes phase is called saturation pressure.
Some Important Concepts for Pure Substance
Melting
It is also known as liquefaction point, it is a point at which solid starts to melt into liquid at atmospheric pressure under high temperature.
Heat energy called the latent heat of fusion is used to convert solid into liquid form.
Freezing
It is the temperature at which a liquid changes phase to solid.
Supper cooled means the liquid is cooled well below its freezing point.
Sublimation
It is a process where a solid turns into a gas without going through the liquid phase. Ex camphor, naphthalene.
Vaporisation
It is the process of conversion of liquid into a gaseous form.
| Evaporation | Boiling |
| It takes place at all temperatures. | It takes place at a specific temperature. |
| It takes place on the surface. | It happens as a bulk phenomenon. |
Condensation
It is a phenomenon in which the gaseous phase is converted into the liquid phase.
Latent heat
It is heat provided to matter during which temperature remains the same but phase change takes place.
Sensible heat
It is heat provided to matter during which temperature change takes place but the phase remains the same.
Physical and chemical changes
Pure substances go through both of these processes so it becomes important to understand them.
Physical change
In this process, only physical appearance goes through change but not its specific composition. Whenever phase change takes place then it is a physical change.
Characteristics
- They are temporary in nature.
- New substance are not created.
- The mass doesn’t go through change.
- Mostly all processes are reversible.
- Change of physical state, appearance, size is seen.
Ex dissolving salt in water, heating liquid.
Chemical change
The identity of the original substance goes through a transformation and a new specific substance gets formed.
Characteristics
- These are permanent changes and hence cannot be reversed to obtain the original substance.
- Possibility of formation of one or more substance.
- Change of mass is definitely happens.
- Composition of final product is different than that of initial product.
- Chemical changes always happen in the company of change in energy.
Ex burning of methane, rusting of iron.
Conclusion
Hence, we have got a basic idea about pure substance, its concept, definition, various examples.
Any doubt, please let us know!
Further Study
Check out our few most viewed articles,

