The zeroth law of thermodynamics is one of the basic laws in our study. From this law, we will learn, how temperature drives thermal equilibrium between objects. Here, we will describe the details of the law, along with examples, applications. Let’s explore the law!
Zeroth Law of Thermodynamics Basics
The zeroth law of thermodynamics can be illustrated with a simple example. When we have a fever, we visit the doctor, and the doctor uses to check our body temperature with a thermometer. Do you know what is thermometer? The thermometer is a device that helps to measure the temperature of the body by using a mercury column.
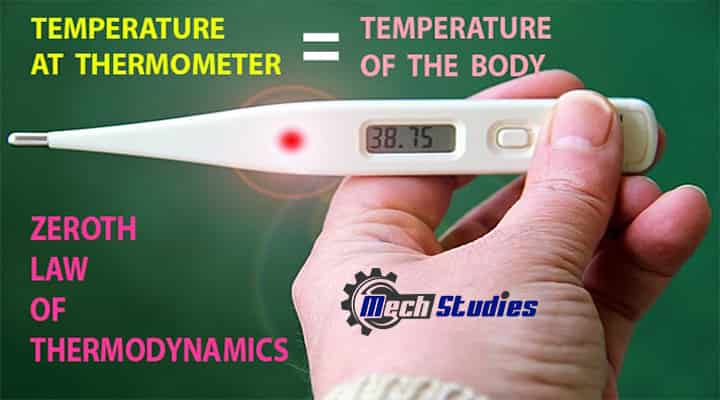
When the thermometer comes in contact with the body, it takes to heat and makes an equilibrium with body temperature. Due to this equilibrium in temperature, doctors are able to measure the temperature of our body. Hence, the body temperature and temperature of the thermometer will become the same and from this philosophy, the concept of the zeroth law has appeared.
What is Zeroth Law of Thermodynamics?
Primary Concept
The zeroth law of thermodynamics is totally based on thermal equilibrium. Hence, the basic concept of thermal equilibrium is necessary. If two systems are placed in thermal contacts, energy may transfer and will come in equilibrium. In thermal equilibrium, there will not be any heat transfer between the system.
Every system is subjected to many surrounding factors like air temperature, pressure, etc., Now, when we say, a body is in equilibrium? Simple! If a body doesn’t undergo any changes its thermodynamic variables like pressure or volume or temperature etc., we call that, the body is in equilibrium.
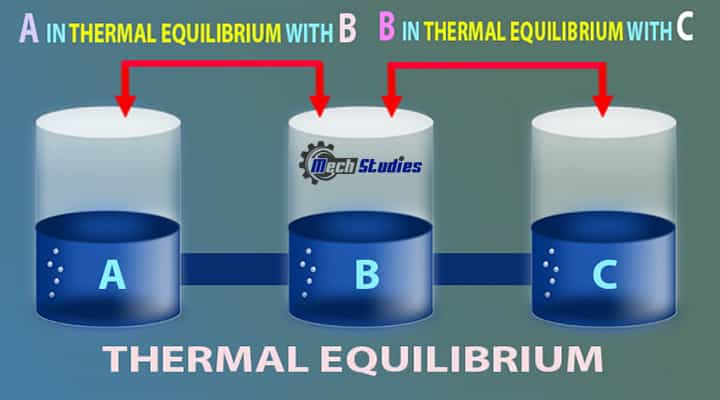
Three liquids at different temperatures are kept in three different containers. All three containers are connected and after a certain time, all liquids will be at the same temperature. This is due to thermal equilibrium.
Zeroth Law of Thermodynamics Statement
There is a total of four laws in thermodynamics and the zeroth law of thermodynamics is the first one. This law states the thermal equilibrium as well as temperature.
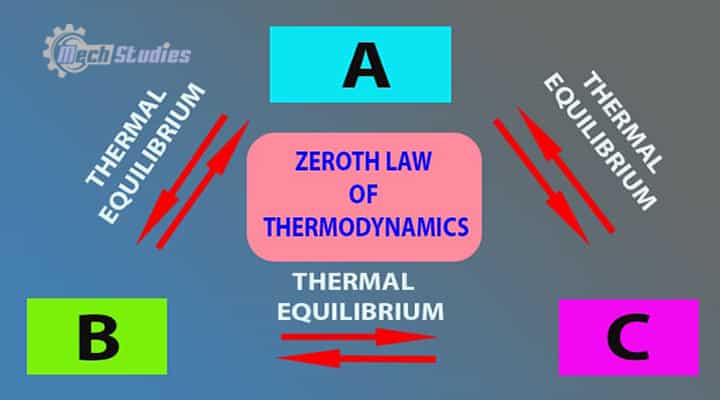
The law is:
The zeroth law states that if two thermodynamic systems are each in thermal equilibrium with a third one, then they are in thermal equilibrium with each other.
Zeroth’s law basically implies the concept of temperature.
History of Zeroth Law
As per to Arnold Sommerfeld, Ralph H. Fowler has invented ‘the zeroth law of thermodynamics’ when he was discussing the 1935 text of Meghnad Saha and B. N. Srivastava. They assume that temperature is a physical quantity and apply the zeroth law of thermodynamics.
The Zeroth Law says that this temperature, defines the direction of heat flow, and it does not depend on the amount of energy that’s involved directly.”
The temperature of the two systems is the only thing you need to know in order to determine the direction of heat flow between them.”
Equation of Zeroth Law of Thermodynamics
Temperature is the main parameter that predicts,
- Whether the heat will flow or not,
- If heat flows, which direction it will flow?
Zeroth law predicts the direction of heat flow and it states that if two systems are in thermal equilibrium, there will not be any further heat transfer. Now, no heat transfer means no change in temperature. So, the final temperature of both the system will be the same. Let’s consider, any three systems,
- System A
- System B
- System C
Now, if System any two systems are in thermal thermal equilibrium with a third system, then all the systems will be in thermal equilibrium. If the System A is in thermal equilibrium with System B and System A is in thermal equilibrium with System C, then, as per zeroth law, System B and System C will also be in thermal equilibrium.
If,
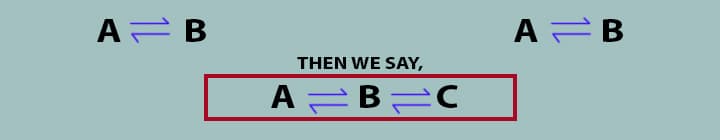
Remember Many times if two objects may not be in physical contact, but heat can flow by radiation.
Why it is Called the Zeroth Law?
There is a very interesting story about calling zeroth law. In the 18th century, few scientists were realized that another law should be there apart from three thermodynamics laws to complete the concept.
- At the same time, Ralph H. Fowler invented a new law of thermodynamics.
- This new law represents a formal definition of temperature,
- It relates all three laws and it should be placed in the first position.
- So, there was confusion since all other laws are already placed in first, second & third position.
- Finally, Ralph H. Fowler, came up with the zeroth law idea to avert confusion.
- Finally, this law was established as the zeroth law.
Zeroth Law of Thermodynamics Examples & Explanation
Description
The law is important for the mathematical formulation of thermodynamics. This law gives a simple mathematical definition of temperature. This law is mostly used to compare the temperatures of different objects. Do you want to measure the accurate temperature?
Take a reference body and consider a few specific characteristics of that body which changes with temperature. The change in that characteristic may be taken as an indication of a change in temperature. The specific characteristics are known as thermodynamic properties.
Zeroth law of Thermodynamics Examples
Since the zeroth law of thermodynamics deals mainly with temperature, one of the main application of this law is in thermometers. We all know, the thermometer is used to measure our body temperature when we have a fever. Now, do you know how does a thermometer work? Let me explain! In the thermometer, there are few things,
- a glass bulb
- a glass column
- liquid, normally mercury
- a temperature scale on the glass tube (it may be in degree C or degree F etc.)
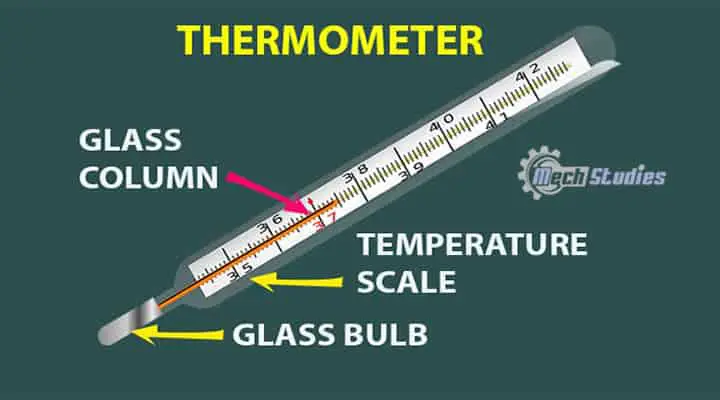
Explanation
Normally mercury is stored in the glass bulb and this glass tube is connected to the glass tube. At normal temperature, mercury is in the bulb only. When the thermometer bulb comes in contact with our hot body, mercury in the bulb takes that heat. Mercury has a property to expand rapidly with a slight temperature difference.
The area of the tube is constant and the mercury starts to expand within the tube. Based on the mark on the tube, we can understand the temperature. Here, mercury takes the temperature from the body and makes a thermal equilibrium. Then get the temperature of the thermal equilibrium. This is simply as per the zeroth law of thermal equilibrium. There are various thermometers used to measure various thermal properties.

- Digital Thermometer
 – placed under the tongue & measure temperature
– placed under the tongue & measure temperature - Electronic Ear Thermometer – measure temperature by infrared technology
- Constant volume gas thermometer – measure pressure
- Forehead thermometers – measure temperature
- Constant pressure gas thermometer – volume
- Pacifier Thermometer- temperature
- Electrical resistance thermometer – resistant
Application of Zeroth Law of Thermodynamics
There are so many ways, the zeroth law is used, these are, as follows,
- When we get very hot food, we wait to make it normal. In this case, hot food exchanges heat with surrounding and bring equilibrium.
- We keep things in the fridge, and the things come in equilibrium with fridge temperature.
- Temperature measurement with a thermometer or another device.
- In the HVAC system, sensors or thermostats are used to indicate the temperature. It always comes in thermal equilibrium with room temperature.
Limitation of the Zeroth Law
There is a certain limitation of the Zeroth law,
- It does not tell us about the direction in which heat flows when they are in contact.
- When two bodies come in equilibrium conditions, this law is unable to tell about the final temperature or the temperature of the equilibrium conditions.
- It does not tell about energy conservation.
- Zeroth law predicts whether there will be heat transfer between objects or not.
- If two objects are not in physical contact, there may also be a heat transfer. For example, if two objects with different temperatures placed little distance, there may be a heat transfer by radiation.
- In the zeroth law, there will not be any heat transfer, if the systems are in thermal equilibrium.
Conclusion
We have learned the ‘Zeroth Law of Thermodynamics’ along with explanations and examples. I Hope, the idea of thermal equilibrium is clear. Let’s see many interesting articles,

